Calmodulin - a Ca++ binding protein
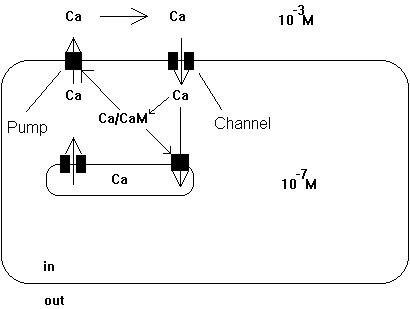
Calcium is a major regulatory cell component and its free concentration in the cell is tightly controlled at 10-7 M. Any changes in concentrations away from this resting level are functionally significant (calcium waves). Prolonged increase in internal Ca++ concentration has been linked to cell death. For example, elevation of Ca++ concentration triggers muscle cells to contract or stimulates neuronal cell to release neurotransmitters from the synapses (see acetylcholine release to stimulate ACh receptor post-synaptically). The cell has specialized storage devises, mostly organelles, but Ca++ also binds to all kinds of proteins, metabolic intermediates, and membranes due to their negatively charged headgroups. The internal Ca++ storage and external high Ca++ levels serve as reservoir to quickly rise internal Ca++ levels or restore Ca++ level after an event of Ca++ depletion. Membrane proteins called Ca++-ATPases are responsible to pump Ca++ ions into these storage organelles and from the inside to the outside of the cell membrane.
Calmodulin (CaM) is a small cytoplasmic protein of 148 amino acid residues. It folds into two domains that are connected by a central seven-turn a-helix which serves as flexible tether connecting the two domains. Calmodulin has 4 Ca++ binding sites, two in each domain. The binding site is located in a helix-loop-helix motif, also called the E-F hand. This motif occurs also in other Ca++ sensing proteins with known structure. The Ca++ ion in each case is octahedrally coordinated by oxygen atoms from the peptide backbone and side chains of the loop as well as protein-associated water molecules.
Ca++ binding to CaM domains (Km ~ 1m M) induces a conformational change that exposes a methionine rich hydrophobic patch which can bind to other proteins. This proteins are known to be Ca++ regulated through their interaction with Ca++ activated calmodulin. Calcium ions do not bind directly to these proteins but exhibit their effect through the mediation of CaM. The CaM binding domains in these Ca++ regulated proteins are basic, amphipathic a -helices. There is no sequence homology found in these binding domains, yet they are structurally homologous.
Fig. Structural changes in calmodulin upon peptide binding
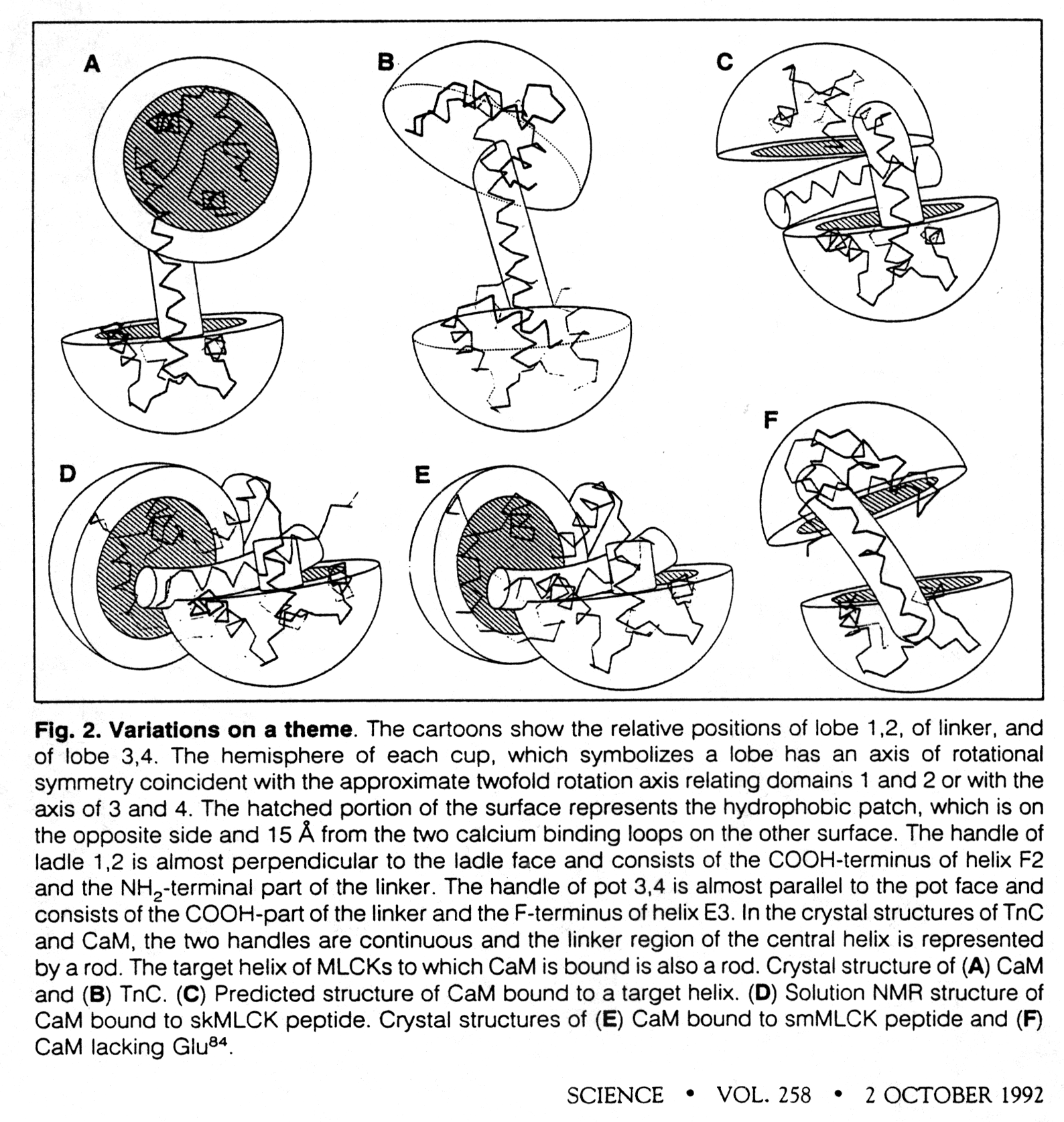
from Kretsinger, 1992
Ca++-ATPase
The Ca++-ATPase or Ca-pump constitutes a family of integral membrane proteins that restore the internal Ca++ storage to resting levels (after muscle contraction; neurotransmitter release) by pumping Ca++ ions across the membrane. The pump uses the energy from the hydrolysis of ATP. The regulation of the pump is controlled by internal Ca++ concentration mediated by Ca++/CaM interaction. The Ca-pump activity constitutes a regulatory feedback loop where the substrate activates its own enzyme. When the cytosolic Ca++ levels are too high, the Ca-pump is activated and move Ca++ ions from the cytosol to the outside or into organelles, mostly endoplasmatic reticulum and mitochondria. The Ca-pump restores a calcium ion concentration gradient of ~104 with [Ca++]out = 1.5x10-3M and [Ca++]in = 10-7M. The pump is self-inhibited in the absence of Ca++/CaM complex. Ca++/CaM activates the ATPase by binding to an inhibitory peptide segment of the pump.
Fig. Control of cytoplasmic Ca++ concentration