Membranes as Therapeutic Targets
The importance of membranes Human
Disorders Membranes are an integral part of life defining cellular structures that have evolved to compartmentalize metabolic activity, protect cells and organs, and provide means of communication over long and short distances. The activity of membranes can be broadly classified as signaling (communication), transport (exchange), and providing structural coherence and functional independence of cells, tissues, and organs. In complex organisms like human beings, the function of membranes is crucial for development, metabolism, motility and reproduction. Signaling is important for sensory organs, but also defense (immune system), and overall metabolic integration (nervous and endocrine system). Sensation and regulation are of course dependent on enzymatic reactions and transport of metabolites throughout the body. Absorption, distribution, energy conversion, and secretion are part of a metabolic cycle that extracts energy and matter from the environment for the benefit of a stable, ordered multi-cellular organism. The maintenance of order based on a steady flow of matter and energy is referred to as metabolic homeostasis. To keep the trillions of cells in a stable form, extracellular protein and carbohydrate components are anchored in cell membranes contributing to cellular adhesion (junctions, connective tissue) forming a structural framework (scaffold) important for development, growth, tissue integrity and regeneration. Membrane proteins contribute to human disorders by causing faulty regulation, transport, or cellular integration of tissues. This can be the result of mutations (hereditary diseases), damaged proteins, or susceptibility to toxins. There are four distinct ways mutations can affect the proper functioning of membrane proteins: 1. defective synthesis (transcription, translation) In addition, some cell surface proteins are the target of antibodies in autoimmune diseases. This leads to the attack and destruction of the cells and tissue expressing the corresponding proteins. Examples are Myasthenia Gravis (read more about it @ eMedicine), a destruction of neuromuscular junctions targeting the nicotinic acetylcholine receptors, or Pernicious anemia (read more about it @ eMedicine), an autoimmune disease of the stomach lining targeting the H/K-ATPase (pump) in gastric pit epithelia. The resulting loss of parietal cells means reduced levels of intrinsic factor, a binding protein of dietary cobalamine, leading to vitamin B12 deficiency. Diseases involving membranes are either the result of unregulated signal transduction pathways or defective transport systems. Antagonists are the most common drug class targeting membrane proteins that are integral parts of such pathways. Therapeutic intervention of antagonists works by blocking ligand binding sites on receptors or interfering with the reuptake of signaling molecules (e.g. Prozac) to prolong the effect of a hormone or neurotransmitter. Diseases related to transporters, however, usually require restoration of function rather than suppression of an enzyme gone wild. This is undoubtedly a more challenging approach for drug development, as rescue of function is not as easily achieved as inhibition of function. In many cases, replacement of faulty proteins by gene therapy or protein replacement may be the only solution. In some instances, however, small peptide mimetics that function as folding templates for misfolded proteins in the endoplasmatic reticulum (ER) can rescue defective proteins and support their transport to the cell surface. An example is IN3 a peptide mimetic that has been shown in vitro (cell culture) to rescue the cell surface expression of Gonadotropin releasing hormone receptor (GnRHR), a member of the G-protein coupled receptor family (P.M.Conn et al., 2002, Mol Intervention 2:308). Eukaryotic cells have multiple membrane
structures separating compartments with functional specialization.
Membranes serve as gatekeepers of the flow of information and metabolites
between compartments within cells as well as between cells and their
surroundings. In multicellular organisms, adjacent cells attach
by forming junctions that can be permanent and last throughout the
life of an organism, or temporary ones that are forming for specific
periods of time during development, growth, wound-healing, or immune
defense. Cells come in a variety of shapes and metabolic functions,
differing in their structural organization with usually asymmetric
shapes. These differences are the result of distinct temporal expression
and spatial distribution of identical or closely related proteins.
The employment of the same members of protein families in different
cell types explains why hereditary diseases caused by a mutation
of a transporter can show neurological, immunological, and metabolic
defects. Cystic
fibrosis (CF @
eMedicine), a defect in epithelial chloride transport, affects
different organs including the lung, intestine, pancreas, kidney
and sweat glands. Most membrane bound processes involve the coordinated activity of several different types of membrane proteins. A well understood 'complex' system is the electrical excitability of membranes. Action potentials are the result of the orchestrated activity of several types of ion channels with distinct gating properties and ion selectivities. An other example of coordinated activity of channels is the control of insulin release form the pancreas. Glucose influx into beta-cells leads to ATP dependent K-channel activation. The resulting depolarization opens a voltage gated Ca-channel. Calcium influx triggers vesicle fusion and the release of insulin. An autocrine feed back mechanism via the insulin receptor stimulates additional insulin synthesis priming the beta-cells for a next round of glucose mediated hormone release. It should be noted, that the blood serum concentration of most metabolites is carefully regulated and maintained within a narrow physiological range. Besides glucose, the homeostasis of nitrogen levels is the most important integrative factor in metabolism. Nitrogen containing compounds are mostly amino acids, with many minor substances (lipids, nucleic acids, specialized carbohydrates on cell surfaces) and the body maintains a tight balance between dietary nitrogen (protein), internal nitrogen that is recycled (tissue protein), and secretion of nitrogenous compounds (urea, ammonia, uric acid). The amino acid glutamine is the central metabolite that is regulated and plays an important role in coupling amino acid metabolism with that of sugar and fat. Needless to say, many membrane transport systems are required for absorption, distribution, and secretion of nitrogenous compounds. Liver and kidney cell membranes play an important role in this homeostatic mechanism. To get the right appreciation of these membrane processes, it is necessary to have a rudimentary understanding of cell membrane structure and its components, the lipids and proteins. Detailed descriptions can be found in accompanying lectures on this site. To briefly summarize, membranes are composed of bilayers of phospholipid monolayers. Membranes thus form elongated, flat sheets with a water soluble, charged surface on each side separated by an internal hydrophobic layer. This hydrophobic layer is made of fatty acids and cholesterol (the latter found only in animal membranes) and functions as an electric insulator and barrier against all water soluble molecules, samll and large. This water soluble molecules can only cross cell membranes with the help of membrane proteins that function as transporter, facilitating diffusion or requiring chemical energy to actively push them across the barrier. The basic activity of membranes is that of a fluid-mosaic structure that is fairly stable, yet highly dynamic and promotes lateral diffusion of its components from one side of a cell to another. Mobility is a function of size of the molecules - the larger the slower, resulting in rapid lipid mobility, but slow membrane protein movement. The latter are often organized in large complexes and clusters reducing the mobility to near zero, allowing permanent anchoring on the cell surface. Consequently, immobile membrane proteins can associate and organize underlying cytoplasmic structures like the cytoskeleton fiber network. The latter mechanism has enormous impact on the overall structure and activity of a cell. Due to the dynamic of its lipids components, membranes can also fuse together when put right next to each other. Membrane fusion (and its reverse, membrane vesicle budding) are important cellular processes. Because of their importance, fusion is not random but tightly controlled by fusion proteins that gives cells control over timing and location of fusion. Endo- and exocytosis are the most important physiological mechanism involving these fusion events. In the laboratory, entire cells can be tricked into fusing their cell membranes producing artificial cells and cell hybrids. The general architecture of membrane proteins provides two distinct forms; first, transmembrane or integral membrane proteins; second, peripheral membrane proteins that are anchored by lipid residues (e.g. G-proteins) or attached by electrostatic interactions (e.g. mitochondrial cytochrome c). The structures of many membrane proteins (30+) has been solved since the first such report in 1985 of the bacterial photosynthetic reaction center. The number of structures, however, is still very small compared to the number of structures of water soluble (so called globular) proteins that ranges in the 10,000. The difference is due to the difficulty of handling the water insoluble membrane proteins under experimental conditions suitable for structure analysis. The hydrophobic characteristic and the structural feature of many membrane proteins (being exposed to the cell surface) makes them susceptible to toxins and drugs that affect membrane function. General and local anesthetics are prominent examples of small molecules that have inhibitory effects on the electrical properties of muscle and neuronal membranes, thus their ability to numb (local) or render us unconscious (general). General anesthetics are hydrophobic molecules of various structure that preferential bind to the lipid portion of membrane and through some not yet fully understood mechanism suppress brain activity regarding awareness and pain sensation. Local anesthetics, however, are water soluble molecules that bind to certain membrane proteins, called ion channels, interfering with their ion conduction property. Thus, they suppress action potential dependent cell function suppressing neuronal signaling of sensation. Natural toxins have long been used as pharmacological agents to purify and study channels. Many naturally occurring molecules found in venom of snakes and spiders have helped us understand the mechanism of the inhibitory function of drugs. These toxins are potent inhibitors of channels and pumps or sometimes overstimulate them, causing muscle cells to stop cycling through contraction and expansion. Proper dosage can turn toxins into useful modulators of channels and pumps. Cardiotonic steroids are examples of naturally occurring drugs that affect the Na/K-pump and thus the baseline ion gradients necessary for proper action potential activation and duration. Signaling disorders Tyrosine
Kinase Receptors Signaling is important for integration of metabolism and coordination of organ activity and defense. Sensory organs, the nervous, endocrine, and immune system make extensive use of signaling processes. An important aspect of signaling is direct cell-to-cell communication regulating cell adhesion and migration, differentiation, growth (development, cancer), cellular immunity and blood clotting. At the center of signaling processes are cell surface receptors and receptors on organellar membranes. Some signaling molecules can diffuse across the hydrophobic membrane due to their low water solubility properties binding to cytoplasmic and nuclear receptors. Signaling via cell surface receptor regulates four major physiological processes. They are: 1. Gene expression activity Receptors are integral membrane proteins with large extracellular and cytoplasmic domains. As is true for any protein system, receptors have an intrinsic internal flexibility that is exploited to regulate the activity of cytoplasmic domains in response to a ligand binding event on their extracellular domains. This process is the canonical receptor mediated activation of a cytoplasmic signaling cascade and known as transmembrane signaling. Such cascades are amplifying a signal where a single ligand-receptor binding event can stimulate dozens of first responses (activate several G-proteins, phosphorylated several kinases) which in turn can each activate another dozens of secondary targets and so on. The result is a single hormone activating hundreds of enzymes. Mechanistically, an extracellular ligand activates a integral membrane protein that changes its cytoplasmic activity through an allosteric mechanism. Allosteric mechanisms are due to protein conformational changes upon ligand binding, affecting enzyme activity or secondary ligand binding on the same protein complex. Models of receptor activation usually assume a two state model with an active and an inactive form. In biochemistry, a receptor can switch between both forms and this process is described as an equilibrium. The important point is the control over this equilibrium, that is to say, how many receptor units in a population at any given time are in one or the other state, active or inactive. Ligand gated receptor are most of the time in an inactive conformation. For most receptors, this is a monomeric form, a single copy of a receptor unit. Ligand binding stimulates dimerization with the dimer being the active conformation. We say that the equilibrium is shifted from one to the opposite state. A typical activation scheme is shown for the tyrosine kinase receptors which are the targets of growth factors and hormones. Dimerization of the extracellular domains results in dimerization of a kinase domain on the cytoplasmic side of the membrane causing autophosphorylation of tyrosine residues. Phosphorylated receptor dimers will bind target proteins and phosphorylate starting a cytoplasmic signaling cascade. The phosphorylated domains recognize adapter modules on target proteins. These adapter modules are either SH2 domains which bind Tyr~P, or SH3 domains which bind proline rich domains. Binding between kinase and target protein brings the latter into contact with the active site of the kinase leading to phosphorylation of the target proteins. An other known adapter module is the PH domain (Pleckstrin homology) which mediates membrane contact through binding to phosphorylated phosphatidylinositols. Dimerization is not necessarily an activation control mechanism. In protein tyrosine phosphatases (PTPs) the monomeric cell surface molecule is the active conformation resulting in the dephosphorylation of P~tyrosine residues. Dimerization of PTPs leads to their inactivation. It can be hypothesized that PTP monomers can disrupt dimers of tyrosine kinase receptors. Consequently, phosphorylation is reduced, while dephosphorylation activity is increased. Down regulating the number of PTPs will allow increased kinase dimers (causing overall signaling), while down regulating kinase receptors will cause PTP dimers (causing overall signal inhibition). An another example where dimerization plays a role in controlling signaling are the G-protein coupled receptors (GPCRs). Here, monomers are mostly the active form, although some types (e.g. GABA receptors) are found to form functional heterodimers. GABA(B1a)-GABA(B2) heterodimerization has been shown to be necessary for cell surface expression. In other cases, dimerization leads to different ligand specificity or as compared to the monomeric conformation. For the beta-adrenergic receptors, monomers are the active form and dimerization has been associated with inactivation by recycling the receptor or removing them permanently from the cell surface through endosome mediated degradation.. Due to the lack of high resolution structures of ligand gated receptors, the details of allosteric activation are not fully resolved. Some enzyme systems, however, have shed light on the model of allosteric regulation. The positive cooperativity of oxygen binding to hemoglobin tetramers is an example thanks to the availability of crystal structures of both a completely oxygen free (T-state) and a fully oxygen saturated structure (R-state). Differences in subunit organization (quaternary structure) and back bone folds in each subunit at both the heme (ligand binding site) and subunit contact sites largely explain the conformational change and the stability of both R and T-state tetramer conformations. An other example is the ras GTPase (related to the alpha subunits of G-proteins) with a GTP (active) and GDP bound (inactive) high resolution structure. G-protein coupled receptors form a large and diverse group of membrane proteins that share a simple seven transmembrane helix motif. The structure of this membrane domain has been solved from X-ray crystallography of rhodopsins - both the distantly related bacterial proton pump bacteriorhodopsin, and the light sensitive rhodopsin from bovine retinal cone cells. No structure of a related ligand binding GPCR is available. In the absence of high resolution data, but availability of sequences and close similarities in membrane spanning domain architecture, modeling of many receptors based on the backbone conformation of bovine rhodopsin helps understand ligand binding diversity of the G-protein coupled receptor system. Extracellular and intracellular loops are the primary binding domains, with the transmembrane helices also serving as binding cavity for small ligands such as acetylcholine, dopamine, glutamate, epinephrine (adrenaline), or serotonine. Approaches of modeling related receptors based on the availability of structures from a member of a protein family is an important novel strategy in post-genomic analysis of biological systems. Thanks to the domain module architecture of proteins, and the conservation of domains in proteins with diverse function (e.g. immunoglobulin domains in tyrosine kinase receptors), the structural conservation can be exploited to predict structures or functional association to novel gene sequences based solely on patterns of similarity in databases. Similarity can be based on sequence similarity (classic BLAST algorithms), similarity in motif, domain, or tertiary structure (proteomics and structural genomics), or similarity in genomic organization (comparative genomics) and co-expression with related genes that form part of a pathway (functional genomics, metabolomics). Many diseases are related to lack of binding, surface expression, or G-protein coupling of these receptors. Diseases can also be related to malfunctioning G-proteins and some bacterial toxins are known to overstimulate G-alpha subunits through covalent modification and constitute activation of effector activity (cAMP production). Cholera toxin stimulates Gas type subunits causing diarrhea and pertussis toxin activates inhibitory Gai subunits, a major cause of whooping cough. The specificity of these toxins for either alpha type has been instrumental in analyzing the effector diversity of G-proteins and comprises an excellent example of biochemical analysis of parts of a signal transduction pathway. An example of GPCR linked disease is congenital nephrogenic diabetes insipidus, where kidney loose response to vasopressin or antidiuretic hormone (ADH). Here, the vasopressin 2 receptor (VR2) can be inactive when mutations cause a it to loose hormone binding, G-protein coupling, or surface expression. The latter can be classified as a misfolding disease where the receptor is most likely stuck in the ER membrane. Some examples of rescue therapies for misfolded proteins have been reported where small molecule templates such as peptide mimetics can bind to the newly synthesized protein and assist it in proper folding. With intense scrutiny of a system often come novel insights. G-protein coupled receptors have been identified to exhibit activities that do not include G-proteins, but depend on the presence of oligomerization domains. The domains are usually short, highly conserved amino acid sequences that mediate specific interactions between proteins resulting in large complex formation. Many membrane associated signaling complexes are know being studied. One example is the post-synaptic organization of glutamate regulated long term potentiation. Here, metabotropic and ionotropic glutamate receptors are coupled to a mediator protein (shank/proSAP) which contains multiple interaction domains, including a PDZ domain, Homer, Ankyrin, and an SH3 domain. As a mediator, this protein organized a membrane bound cluster connecting the cell surface with cytoskeletal actin filaments. Homer domains have been identified by studying the coupling of metabotropic glutamate receptors with the calcium release channel of the endoplasmatic reticulum. Here, homer protein dimers mediate the direct protein-protein interaction between the cytoplamsic and ER membrane by binding to both the mGluR and Ryanodine receptor. A member of the homer family that is unable to dimerize can be upregulated and competes with homer dimers, thus counteracting the plasma membrane to ER membrane coupling. The function of PDZ domain has been shown for beta-adrenergic receptors that bind via a C-terminal DSLL motif to a PDZ domain of the sodium-proton-exchanger regulatory factor (NHERF) providing a G-protein independent control of electrolyte homeostasis by epinephrine. In yet another G-protein independent pathway, beta-arrestin has been implicated in controlling localization of signaling complexes rather than just receptor desensitization. Beta-arrestin is involved in the regulation of c-Src family tyrosine kinase activity in response to chemokine stimulated degranulation in neutrophils. Beta-arrestin controls a complex formation of chemokine receptor with a tyrosine kinase receptor and through vesicle mediated trafficking of CXCR1 (a G-protein coupled chemokine receptor) directs the co-localization of tyrosine kinase receptors with granule vesicles. Such a coupling of GPCR internalization and activation of tyrosine kinase receptors can be viewed at as the timing of neutrophil activation where chemokine sensitivity is inactivated and degranulation activated making neutrophil activation irreversible. Signaling is often understood as the result of a diffusible ligand activating its receptor. However, a no less important form of receptor signaling involves the use of non-diffusible ligands. Unlike neurotransmitters, hormones, and growth factors, a diverse group of cell surface molecules recognize other cell surface molecules. Either homotypic or heterotypic recognition is possible. The resulting cell adhesion and formation of various types of permanent and temporary junctional complexes is important for development and differentiation. Tight junctions, desmosomes, and gap junctions provide both tissue integration as well as cell to cell communication. Temporary junctional complexes have recently been described between T-cells and antigen presenting cells (APCs) forming immunological synapses that regulate T-cell maturation. Lack of proper cell adhesion can cause cancer growth and metastasis, a process of cell migration with subsequent binding and proliferation of cancerous tissue. Four major classes of cell adhesion molecules have been characterized. They are: 1. Cell adhesion molecules (CAMs) Cell adhesion molecules or CAMs are mostly expressed in cell type specific patterns and regulate homotypic cell recognition in a calcium dependent manner. Lack of these CAMs results in loss of cell contacts and potentially cancer and their presence is guiding adjacent cells to form stable contacts and differentiate into tissue specific cells. The best known type is E-cadherin that is specifically expressed to mediate homotypic association between cells, particularly epithelial cell layers (hence the E). E-cadherin has first been shown as uvomorulin to control morula compaction in early development. The major location of uvomorulin (E-cadherin) is the intermediate junction in the tripartite structure between adjacent cells in epithelia. The three junctions found there are the tight junction towards the apical membrane (e.g. lumen of the intestine), followed by the intermediate junction and the desmosome structure located toward the basolateral side of the epithelia. Cadherins form intermediate junctions by inducing a zipper like complex between multiple E-cadherin dimers on each cell surface. Cadherin clustering is followed by intermediate filament assembly (alpha and beta-actinin, plakoglobin) and actin skeleton organization. Ig-CAMs are more diverse and function in a calcium independent fashion. They also can mediate homotypic as well as heterotypic cell adhesion. Splice variants of Ig-CAMs have been described that provide different ligand specificity and different cell membrane anchoring. A shift from transmembrane to GPI anchor (an extra cellular lipid anchor of cell surface proteins) allows CAM binding domains to rapidly diffuse along cell surfaces or to be released from its anchor through phospholipase mediated hydrolysis. Thus, cells can rapidly switch from adhesion to loss of adhesion or provide soluble ligands that can interfere with neighboring cell adhesion events. Integrins are a heterogeneous class of cell adhesion molecules that mediate signaling between extracellular matrix (ECM) components and the cytoskeleton. Integrins operate as heterodimers of alpha and beta subunits. From a selection of 19 alpha and 8 beta subunits, 24 functional alpha-beta dimers have been described so far. The diversity of integrin dimers is related to diversity of function that goes beyond ECM interaction. Integrins can also mediate heterotypic cell adhesion and are involved in dynamic processes that do not need long time contacts. Integrins control signaling pathways of the ERK, MAP kinases affecting gene expression needed for differentiation, proliferation and migration of cells. They integrin mediated ERK pathway can interact with GPCR coupled ERK signaling demonstrating cross-talk between cell surface receptors. Integrins also signal via Rho GTPases directly to the cystoskeleton regulating stress fiber growth, and the growth of microfilaments in filapodia and lamellipodia. Filopodia are required as sensory structures and effectors of motility. They receive signals from the molecular environment and transmit signals that alter the cytoskeleton and cellular motility. Selectins are specialized cell adhesion molecules involved in platelet and leukocyte adhesion to endothelial cells. They play an important role in recruiting cells in wound healing and inflammation. Their ligands are carbohydrate rich cell surface proteins (glucans) or glycolipids. Transient bond formations between the selectins and their ligands mediate the early steps of the adhesion cascade. Selectins contain an N-terminal extracellular domain with structural homology to calcium-dependent lectins such as mannose binding protein. The latter is part of the innate immune system providing broad protection against pathogenic microorganisms by recognizing their glycosylated surfaces. Selectin expression on epithelial cells causes a a slow movement of leukocytes along the endothelium via transient, reversible, adhesive interactions. Three types of selectins have been described; L-selectin is leukocyte specific, P-selectin is expressed on platelet surfaces, and E-selectin is expressed on cytokine activated endothelial cells. Selectins, integrins, and Ig-CAMs are involved in leukocyte rolling, a dynamic process of cell adhesion mediated between leukocytes and infected endothelial cells. More about the process of inflammation by Klaus Ley and Adam Brooke can be found on-line at the Department of Biomedical Engineering at the University of Virginia. The immunological synapse between T-cells and antigen presenting cells (APCs) is another dynamic system that involves formation of large protein clusters with functional domain organization. This 'synapse' which resembles more a junctional complex involves many different types of cell surface receptors including integrins, Ca-independent Ig-CAMs, and T-cell receptors that form functional contact with MHC-antigen complexes. Synapse formation through receptor clustering occurs within minutes resulting in calcium mediated intracellular signaling in the T-cell, which will be loosing contact and now being activated for proliferation and differentiation. Therapeutic strategies involving cell adhesion molecules:
Dual function proteins have been described that have both cell adhesion domains and a transmembrane domain with transport function. An interesting example is the mechanosensitive cation channel involved in adult polycystic kidney disease or APKD. Another is Lu-ECAM-1 which has also been described as a calcium activated chloride channel (CaCL). Both protein systems show sensitivity towards proteases and separation of the extracellular cell adhesion domain from the channel domain appears to be an activation mechanism in the transport function of these dual function proteins. A specialized junctional complex between adjacent cells is that formed by connexins or gap junction proteins. Gap junction channels cluster into fairly large 'plaques' that can contain thousands of individual channel units. Gap junctions provide metabolic coupling allowing the simple diffusion of small metabolites between cells without them being transported out of cells and back into them. Because gap junction channels are constitutively open (and can be closed by voltage, local anesthetics or lack of calcium) they also provide electrical coupling between neighboring cell membranes. Metabolic coupling can occur over several cells forming metabolic supercompartments called a syncytium (a polynucleated cytoplasm). Similarly, electrical coupling has been shown to coordinate action potential activity in muscle tissue of entire muscle fibers to promote synchronous calcium triggered contraction-relaxation of muscle.
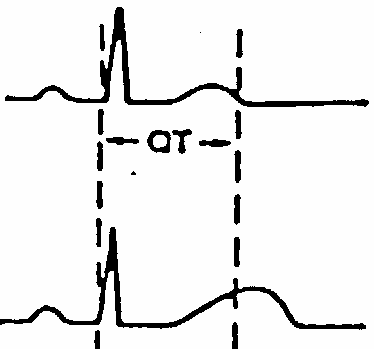
Although gap junction structure and function is fairly well characterized by genetic and cell biological studies and heterologous expression systems, their physiological role eludes clear understanding. In vertebrates some 17 different connexin genes have been identified. Connexins are expressed in every cell type, except single celled erythrocytes. Every cell studied co-expresses at least two types, although their functional and structural differences are not drastic. Knockout studies indeed demonstrate that despite the cell type specific expression, no lethal phenotype arises indicating a strong redundancy in gap junction function. The most interesting aspect of gap junction coupling is the diffusion and local distribution of second messenger molecules. All gap junction channels so far tested show some selectivity, but are not highly specific and thus their commonly large pore makes them equally permeable to nucleotides, sugars, and amino acids and their derivatives. Many diseases related to connexin mutations have been characterized. For example, some mutations (but not others) in connexin 26 (also known as beta2) have been linked to deafness. Interestingly, while these mutation cause disorders in the peripheral nervous system, they do not affect liver function, the organ that strongly depends on the expression of connexin 26. Gap junctions of the inner ear may play a role in maintaining potassium homeostasis, which is important for inner-ear function and, thus, hearing. It has been proposed that mutations in Cx26 may disrupt potassium circulation and result in deafness. A peripheral nervous system disease linked to the other liver resident connexin 32 is called X-linked Charcot-Marie-Tooth (CMTX) disease. CMTX is a group of hereditary motor and sensory peripheral neuropathies caused by demyelinating axons of peripheral neurons. Again, connexin 32 mutations do not affect liver function. Deafness is a complex dysfunction and many different proteins have been identified in different forms of hearing loss or impairment. This includes transcription factors, potassium channels, extracellular matrix proteins, cadherins, tyrosine kinase receptors, an ABC transporter, serine proteases, and an anion transporters. The latter has been found to cause Pendred Syndrome, a hearing loss due to insufficient supply of sulfate ions for bone formation in the cochlea. The transporter, who has been well characterized and is highly expressed in the thyroid where its role is to facilitate iodide uptake, is also permeable for chloride and sulfate ions. Electrical excitability of neuronal and muscle cell membranes allows the rapid transport of information along cell surfaces. This form of lateral signaling is manifest in periodic action potentials leading to cell type specific 'firing' patterns. Chemical and electrical synapses, in turn, transmit the electrical signal across inter-cellular gaps to neighboring cells. Proteins involved in electrical signaling are ion channels and pumps. The former mediate the signal, while the latter restore the used-up ion gradients. Mutation in channels can cause arrhythmias in heart muscle due to changes in channel gating, ion conduction, or abolished function. Many drugs bind to channels and pumps affecting the periodicity of signaling or open times of channels and pumps. Action potentials can be understood in terms of tightly regulated ion channel activity. Action potentials are temporal changes in membrane potentials. At rest, cell membrane have a voltage around -40 to -80mV, meaning the inside of the membrane has fewer positive charges than are found near the outside surface. Voltage changes can be triggered by opening ion channels. Usually, a ligand gated channel (or mechanosensitive channel) opens a cation selective pore facilitating Na+ influx bring more positive charges into the cell (inward currents). The resulting change in potential from inside negative to more positive values is called depolarization and causes subsequent activation (opening) of voltage gated Na-channels bringing even more positive charges into the cell. Next, voltage gated K- and Ca-channels open. The K-channels allow K+ efflux reversing the depolarizing effect of Na influx bringing the potential back to negative resting values. Calcium channels, however, reinforce influx of positive charges (Ca++) and their effect is to modulate the length of the action potential slowing down hyperpolarization by K+ efflux. Importantly, all channels will spontaneously inactivate with varying kinetics. Thus, cell type specific expression of channels with different activation and inactivation kinetics will produce different 'shapes' of action potential, some with rapid increase and decrease, some with slow increase and/or slow decrease. The length and profile of action potential is tuned to physiological needs and any deviation from these 'firing' patterns can cause malfunction of neuronal coupling or the repetitive pattern of muscle contraction - relaxation cycles such as in heart. Many arrhythmias have been traced to mutations in voltage gated channels that alter the length (or period) of the action potentials controlling heart beat. The long QT-syndrome is one of the physiologically best understood hereditary disease underlying heart arrhythmias. For historic reasons, the ion channels of excitable membranes responsible for action potentials have been studied extensively by electrophysiological and pharmacological means. Most of the channels directly involved in electrical signaling are cation selective. However, the existence and involvement of cation selective channels is an important aspect in understanding inhibitory and hyperpolarizing effects on neurons and muscle cells. In other words, chloride channels are well known to suppress electrical activity of excitable membranes. They control the frequency and length of resting periods in neurons and their defects can cause malfunctioning and overstimulation of neuronal activity. Mutations in chloride channels have been linked to diseases. The focus here will be on myotonias and myopathies, muscle anomalies and weakness caused by chloride channel mutations. Other diseases are cystic fibroses, anxiety, renal failure, kidney stone disease, neuropathic pain, and neuronal and liver encephalopathies. Like any other channel system, chloride channels form a large and structurally heterogeneous group with ligand gated (glycine, GABA gated channels in inhibitory synapses) and voltage gated types (ClCs), but include also CFTR, an ATP coupled transporter (see cystic fibrosis). The structure of a bacterial voltage gated chloride channel has been solved. It is a dimer with two channels that can open and close independently but also in coordination. The involvement of alpha helical dipole structures to stabilize permeant ions has confirmed the use of such motifs in ion selectivity in general. The tunneling of ions across membranes can also be compared to substrate tunneling in enzyme complexes that provide efficient throughput of metabolic intermediates in a pathway. A voltage gated chloride channel unrelated to the ClC family and also sensitive to calcium regulation has been identified in epithelial cells on the apical surface. This channel is a dual function protein with a large N-terminal cell adhesion domain, which has been independently identified as Lu-ECAM-1, and Ig related cell adhesion molecule. Expression, molecular weight, and post-translational hydrolysis are all consistent with the dual function of this apical membrane protein. As mentioned above, chloride channels are involved in hereditary diseases of skeletal muscle dysfunction. But many of these diseases have several phenotypes, as different ion channels are involved in electrical excitability. Also the reason for lack of excitability or overstimulation can vary as proteins may have altered activation-inactivation kinetics, ion permeability, or defective cell surface expression. Myotonia Congenita or Thompson's disease is related to a defect in ClC1, but also Na-channels mutations have been identified. An example is Hyperkalemic periodic paralysis (HYPP) where Na-channel mutation give rise to cold induced muscle weakness. Malignant hyperthermia is due to a defect in calcium regulation at the level of the calcium release channel (ryanodine receptor, RYR1). If the calcium release channel stays open longer in the presence of anesthetics, the elevated cytoplasmic calcium levels could result in permanent contraction causing muscle rigidity and paralysis. As the cells try to restore normal calcium levels, they are metabolically active to produce the necessary ATP for the calcium pump. This increased metabolic activity releases heat, excess lactic acid and carbon dioxide. Other myopathies are the result of muscle degeneration or degeneration of synapses between neurons and neuromuscular junctions. Defects in ligand gated receptors that are found in large numbers on synaptic membranes (both pre and post-synaptic cleft) and antibodies generated against those receptors can cause decreased electrical excitability or destruction of synaptic membranes. Myasthenic syndromes are a group of disorders related to many different proteins responsible for cholinergic transmission in chemical synapses; the acetylcholine mediated stimulus of action potentials. Affected proteins include acetylcholine esterase, the nicotinic acetylcholine receptor, but also Na-and Ca-channels colocalized in the endplate membrane of neuronal junctions. Myasthenia Gravis (MG) is due to a reduction in the number of acetylcholine receptors (AChR) on the postsynaptic membrane. Interestingly, many patients with MG have an epsilon subunit expressed which is fetal tissue specific and replaced in adult by a delta subunit. Auto-antibodies against the epsilon subunit may be responsible for the generation of MG. As these degenerations develop over longer periods of times and only partially affect the tissue, the related syndromes can be medicated by adding more signaling molecules. This can be achieved by inhibiting acetylcholine esterase resulting in prolonged presence of acetylcholine in affected synapses. Other neurotransmitters may experience elevated concentration by inhibiting their reuptake mechanism. Transport disorders Classification
of transporters Transport processes designate the flow of molecules and ions across cell membranes. Transport processes are regulated and specific and the result of flux-coupling and synergistic interaction among several transporters. Transport disorders constitute the largest class of membrane associated disorders as they relate to absorption, secretion, homeostasis of metabolite distribution (including water and salt), and energy conversion. The striking compartmentalization produced by cell and organellar membranes makes the need for transport of molecules between those compartments and thus across membranes obvious. Compartmentalization is also a way of controlling metabolic processes by controlling substrate availability and sequestration. Transport proteins carrying ions contribute to signaling processes as well (see ion channels above) and it is not possible to make a clear distinction of where one process begins and the other ends. Transport disorders are caused by defective or unregulated carriers, facilitators, and pumps. Because of these multifactorial systems a defect in any protein involved in a specific physiological process may hamper it. And as many transporters are involved in different transport processes when paired with different partners, a defect in a single transporter may affect more than one type of physiological mechanism. Defective transport not only results in malabsorption, but also accumulation of metabolic intermediates, often with toxic consequences. Other defects play a role in energy coupling (mitochondrial and peroxisomal disorders) or cell volume regulation. Control of water flow is important for solubilization and distribution of metabolites, body temperature regulation through sweating, and waste secretion. Many bacterial toxins negatively affect a cell's ability to regulate water and ion absorption and retention causing loss of water (diarrhea) or the accumulation of metabolites. Transporters are classified according to structural and functional categories to highlight their evolutionary relationship. The two major functional classes are channels and carriers. Channels are distinguished from carriers based on flux rate, with channels having a much higher throughput rate than carriers, which are slower and either depend on energy to pump or facilitate the exchange of larger molecules across cell membranes. Channel proteins are categorized into alpha helical proteins, beta barrel proteins, toxins, and peptide channels. Most channels and those discussed for signaling functions belong to the alpha helical channel protein category. Most toxin channels and peptide channels are also adopting alpha helical motifs. However, they are distinguished from protein channels because they lack regulation and are used for defensive purposes resulting in the death of cells whose membranes they can insert to. Beta barrel protein channels constitute a minor class of channels called porins with largely size selective function and some substrate specificity. They are found in the outer membranes of Gram-negative bacteria. A distant relative is found in the outer membrane of mitochondria (of bacterial origin) which serves as a electrostatic screening of metabolites entering and leaving the mitochondrial compartment. The mitochondrial porin is also known as VDAC, or voltage dependent anion channel, with a cutoff limit around 1kD. Carriers are a more diverse group including primary active transporters (pumps, e.g. Na/K-ATPase), secondary active transporters (symporters and antiporters, e.g. Na/glucose-symporter, SGLCT2), facilitators (uniporters, e.g. Glucose transporter, GLUT2), and group translocators (e.g. phosphotransferase system, PTS).
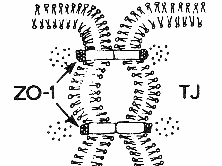
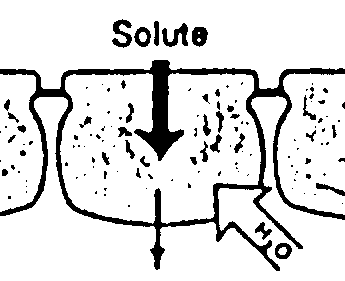
Volume control and concomitant electrolyte homeostasis and metabolite coupled transport across epithelial cells is a major function of transporters in non-excitable membranes. While 'excitability' of membranes refers to the ability to generate action potentials, voltage sensitivity is important, too, in non-excitable membranes, as localized de- and hyperpolarizations are used to control calcium entry and thus exocytosis (e.g. insulin release from pancreatic beta cells) and other signaling processes. Besides calcium channels, chloride channels play a major role in controlling basic electrolyte homeostasis as they allow flow of the major anion to couple ion flux based on charge neutrality. The basic mechanism of volume control is the osmotic property of semipermable membranes that allow the flow of solvent, but not solutes. Thus, unequal solute concentrations in compartments separated by cell membranes result in the osmotic flow of water to equilibrate the chemical potential difference of the two solutions. Water will generally diffuse into the compartment with the higher solute concentration and its volume will shrink accordingly. The compartment gaining water will experience a volume increase. Cellular compartments with varying electrolyte concentrations and mixtures of salts and solutes can adjust osmotically driven flow of both water and solutes by opening and closing water and solute selected transport proteins. Cells can perform transport processes of various kinds through their ability to control and adjust basic solute permeability by regulating gene expression, vesicle trafficking and function of transporters. Transporters increase the rate of diffusion across membranes by several order of magnitudes (channels have the highest increase) and can be kinetically characterized using theories developed for enzyme kinetics. The permeability rates of solvent and solutes across lipid bilayers differs considerably. Although water is a polar molecule, it has one of the highest diffusion rates across membranes in the absence of transporters (permeability coefficient ~ 3x10-3 cm/sec), while polar metabolites like urea and ions have permeability coefficients several order of magnitudes lower (urea ~10-6cm/sec; Na+ ~10-14cm/sec). The recently described water channel family (aquaporins) plays an important role in epithelial transport of solutes in the intestine, kidney, lung, and sweat glands that all need an elevated water flow across these cell barriers to maintain a steady absorption or secretion of polar and charged solutes and to maintain osmotic stability during the process. Aquaporins are water selective channels (some with selectivity for glycerol, an important metabolite linking carbohydrate with phospholipid and fat metabolism) that prohibit the flow of small ions. Aquaporins increase water flow dramatically above background permeability. Measured by the activation energy (Ea) and hydraulic permeability coefficient (Lp), expressing aquaporins in cell membranes decreases the activation energy for water flow by a factor of 3 to 4, while increasing the hydraulic permeability coefficient by a factor of about 20. Vesicle swelling assays with aquaporins have shown the size selectivity and amphipathic property of solute-channel interaction found in the high resolution structure of a bacterial glycerol facilitator (GlpF). Glycerol units, although polar, can adopt an amphipathic structure that allows the solute to optimally interact with an amphipathic channel wall made up of polar residues on one side and non-polar residues on the opposite side of the pore. Nephrogenic diabetes insipidus is the result of defective water reabsorption in the distal duct tubules of the kidney. The disorder can be due to defective aquaporins or lack of regulation of aquaporin expression, under the control of ADH (vasopressin or antidiuretic hormone, see above GPCR). Transport across cell membranes is an important physiological process for the distribution of metabolites and biosynthetic building blocks, but also waste products and secretion of excess material. Many diseases are related to malfunctioning of absorption and secretion or its misregulation causing systemic imbalances leading to deficiencies or toxic accumulation of chemicals. Absorption and secretion in the gut and kidney can best represent the fundamentals of transport mechanisms and their physiological integration and control. The stomach, small intestine, and colon form a segmented organ responsible for the degradation and absorption of nutrients and the secretion of waste products. Different segments have different absorptive mechanisms. The small intestine specializes in absorption of water, amino acids, carbohydrates, fats, vitamins, minerals and drugs. The colon which is rich in bacteria absorbs water, minerals, short chain fatty acids (bacterial products), and drugs. An important function of the colon is to control intestinal fluid balance with the ileum being highly leaky and the distal colon tight. Leakiness is a function of paracellular pathway controlled by tight junctions, particularly the zona ocludens (ZO) controlling epithelial resistance. Tightening of the ZO gives the control over water flow to the epithelial cells and regulated transport systems. The result of increasing tightness of the epithelial mucosa from the ileum to the distal colon is that only a fraction of the water needed to solubilize metabolites and facilitate transport is lost through fecal excretion. Up to seven liters of internal water as part of the entero-hepathic circulation are recycled by the small intestine (secretion and absorption). An additional 1.5 liter of dietary fluids are taken up with only a fraction (0.1 liter) lost in the feces. Extra fluid absorbed by the intestine is excreted in the urine or lost through sweating. The structure of the intestine provides control over uptake and secretion and the surface architecture is optimized to increase the area through multiple folding of the cell layer into villi and cell membranes into microvilli. The average intestinal membrane surface area is estimated at about 300m2. The mucosal cell layer itself is surrounded by circular smooth muscle, connective tissue, blood vessels, lymph, and supporting ligament. These functional specialization of the gut allows physiological regulation of absorption and secretion through neuronal, hormonal, immunological and lumenal signaling molecules. Neuronal regulation of the gut by the Enteric Nervous System (ENS) uses adrenergic pathways controlling absorption and cholinergic pathways controlling secretion. Together, they mediate the interaction between the gut and the brain with sensory neurons, interneurons, and motoneurons (controlling smooth muscle for gut contractions and blood flow) explaining some of the emotional connections between the brain and digestion. The villi structure of the epithelial cell layer provides a functional segregation into absorptive and secretive processes on the tip and crypt of the villi, respectively. The enterocytes on the villi tip are specialized in sodium and/or proton driven nutrient uptake, a classical mechanism of flux-coupling of one solute to the gradient of another one. Both sugars and amino acids depend on the sodium/proton driven uptake into the blood stream. Specialized symporters (Na-glucose SGLT1) on the apical membrane (the intestinal lumen surface) absorb metabolites which accumulate in the enterocyte and diffuse into the blood capillaries through facilitators (e.g. glucose transporter, GLUT2). Secretion of chloride and/or bicarbonate maintains osmotic homeostasis. Defects in transport function or its regulation can severally affect homeostasis and causes excessive water retention or loss. Diarrhea is one of the most common disease and caused by many different factors related to neuronal, immunological, hormonal regulation, or lumenal content, particularly bacterial toxins. Well known (but not necessarily well understood in terms of mechanism) causes of diarrhea are Cholera, Inflammatory Bowel Syndrome (Crohn's Disease), Celiac disease (also known as celiac sprue and gluten-sensitive enteropathy), lactose intolerance, and AIDS related diarrhea. While cholera and AIDS related diarrhea are related to bacterial infections and release of toxins, Crohn's and celiac disease are the result of damaged mucosal epithelia due to overractive immune responses and altered cell adhesion mechanisms. Lactose intolerance and the related glucose-galactose malabsorption result in accumulation of mono- and disaccharides in the intestinal lumen and exert osmotic pressure on the epithelia resulting in the loss of water. Glucose-galactose malabsorption is well understood at a mechanistic level. Here, the apical Na-coupled transporter SGLT1 is defective and cannot be transported to the cell surface. In a functional system, glucose is transported into the enterocyte along with a sodium ion. Glucose then diffuses into the portal blood via the glucose-specific transporter GLUT2. Sodium leaves the enterocyte via the Na/K pump on the basal membrane and potassium is recycled through a K-channel. This energy driven uptake of glucose and sodium serves as a prototype mechanism of absorption and secretion. Many transport defects affecting the gut also affect other organs that rely on epithelial absorption and secretion mechanism. This includes the kidney, sweat glands, and lungs. Renal glucosuria is a condition with enhanced glucose in the urine. The cause is defective reabsorption of glucose from filtered blood serum fluid in the nephrons of the kidney. The defect is related to the sodium coupled glucose transporter. Patients with glucosuria have no intestinal glucose uptake defect, indicating that a different transporter, SGLT2, is expressed in the kidney epithelia. A large number of other diseases are related to changes in transport capacity and specificity. Many of them are clinically well known, although a mechanism and thus cure eludes scientists. A good example is hereditary hemochromatosis. This disease is characterized by an increased iron uptake across the the intestinal epithelia and subsequent accumulation of iron in liver and kidney, having toxic effects on these organs. Different proteins have been isolated with mutations contributing to the disease. This includes iron transporters (divalent metal ion transporter, DMT1), iron transport protein receptors (transferrin receptor, TfR1 and 2), and HFE, an immune system protein closely related to the MHC class I alpha subunit complex. The latter associates itself with transferrin receptor, downregulating iron uptake. Mutations in HFE cause the protein to get stuck in the ER resulting in excess transferrin-transferrin receptor interaction and subsequent iron uptake into cells. The complete mechanism of the regulation of iron uptake with HFE, however, remains somehow elusive. As mentioned for the basic glucose transport in intestinal enterocytes, ion gradients play an essential part in the flow of metabolites. Prolonged flow will dissipate these gradients making them less efficient and thus rebuilding these gradients is essential. This is the function of pumps or ATPases that use chemical energy released from ATP hydrolysis to push ions against their gradient across cell membranes. Some pumps (F-type) work in reverse and use proton flow to synthesize ATP. These ATP synthases are essentially molecular motors coupling proton flow to a rotational mechanism that affects the conformation of enzymatic subunits in a cyclic way promoting condensation reactions between ADP and inorganic phosphate. Pumps are estimated to consume up to 50% of all metabolic energy as ion gradients are important for transport (flux coupling) and signal generation (action potentials and calcium signaling). The primary energy form for all life comes from photosynthesis and light independent electrogenic sources that couple electron transport systems (e.g. mitochondria, chloroplasts, bacterial membranes) to proton pumping. The F-type ATPsynthases convert the proton gradients into ATP and ATP can be used to fuel all metabolic processes, including Na, K, H, and Ca pumps (P-type ATPases). Pumps are highly substrate specific and have a slow kinetic, compared to carriers and channels. In addition to their selectivity, they also display a tightly controlled stoichiometry when pumping more than one ion species, usually in an antiport fashion (exchangers). Thus, the Na/K-ATPases pumps 3 sodium ions for every two potassium ions, while the K/H-pump exhibits a 1:1 stoichiometry. The indicated number of ions are transported for every molecule of ATP hydrolyzed. The hydrolysis is performed in two steps, where the gamma phosphate is transferred to the pump (phosphorylation step), and the phosphorylated enzyme eventually dephosphorylated (completing the hydrolysis step). This two step mechanism where the pump itself is temporarily phosphorylated appear to allow the separation of the countertransport of the ions pumped across the membrane. The dephosphorylated state binds sodium on the cytoplasmic face, and phosphorylation releases the sodium ions from the pump on the extracellular side of the membrane. The phosphorylated, sodium free pump then binds two potassium ions on the outside. Dephosphorylation of the pump transfers the potassium ion across the membrane and releases them into the cytoplasmic compartment. For all their importance in basic membrane energy metabolism, it
is not surprising that defects in pumps are associated to diseases.
Pernicious
anemia is a defect in vitamin B12 absorption. It is an autoimmune
disease of the stomach lining against the H/K-exchanger resulting
in the loss of chief and parietal cells. These cells are the main
lining cells and responsible to control pH and secretory function
of hormones and intrinsic
factor. The latter is a protein that binds cobalamine (Vitamin
B12) protecting it from the acidic condition of the stomach. The
H/K-exchanger can also serve as therapeutic target to treat peptic
ulcer. This ulcer is caused by the bacteria Helicobacter pylori
that thrives at low pH and grows in the gastric pit folds of
the stomach lining. Inhibiting the pump for several weeks will cause
elevated pH values that negatively affect growth of H.pylori. An other class of pumps that relies on phosphorylation from ATP hydrolysis are the ABC transporters (ATP cassette binding proteins). ABC transporters have very diverse functions and show a broad substrate range, unlike they highly specific F- and P-type ATPases. They can transport ions, nucleotides, lipids, peptides, and a plethora of organic molecules with and without charged groups. This class of transporters has been first described for P-glycoprotein and multi-drug resistance mechanisms. It is related to bacterial transporters that play essential roles in metabolite uptake like sugars and amino acids. Interestingly, ABC transporters have conserved domains that are found in DNA binding proteins (associated to nucleotide binding region, ATP cassette). Comparison of these DNA binding proteins and their ligand binding properties (e.g. ligand controlled transcription factors) with ABC transporters have been used to create models of substrate transport in multi-drug resistance. ABC transporters have been implicated in diseases. Cystic Fibrosis (CF) is an imbalance of chloride secretion in epithelial cells where mutation in the cystic fibrosis transmembrane regulator (CFTR) protein cause defective chloride transport across apical cell membranes. Cystic fibrosis is characterized by a slow accumulation of mucus and debris in the capillaries surrounded by the epithelia. Low or defective chloride secretion reduces also water flow. The water flow is necessary to clear capillaries from particles and fibrils that may accumulate. The most common defect in the CFTR transporter is related to a deletion mutation that causes a trafficking defect and lack of transporter on the cell surface. Other mutations cause defective regulation (ATP binding) or chloride conductance. An other defect associated to ABC transporters is called sitosterolemia and is caused by defective sterolins (ABC G5 and G8). Sterolins function to expel plant sterols (e.g. sitosterol) in the intestine, but also have a low affinity for cholesterol. As a result no plant sterols and less than half of dietary cholesterol is absorbed. Defective sterolins cause a loss of control of sterol secretion resulting in unhindered absorption of plant sterols and dietary cholesterol. Cholesterol absorption is easily prevented by omitting cholesterol from the diet. The liver will be able to provide all necessary cholesterol through de novo biosynthesis. The important endoplasmatic reticulum enzyme HMG-CoA reductase is the major target of cholesterol lowering drugs, the statins. A related cholesterol transport defect causes Tangier disease (TD), a genetic disorder of caused by ABC1 transporters that is expressed in most cells and used to secrete excess cholesterol. Bile production in liver depends on the secretion of many organic molecules, some of which are negatively charged. ABC2 transporters are selective for organic anions dubbed multi-specific organic anion transporter (MOAT). Dubin-Johnson syndrome is caused by mutations in the canalicular multispecific organic anion transporter of liver hepathocytes resulting in conjugated hyperbilirubinemia. The latter is a degradation intermediate of heme molecules and will accumulate to toxic levels if the transporter is defective.
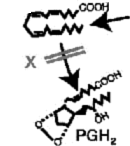
Metabolic disorders of membrane lipids Most membrane bound disorders are recognized as being the result of faulty proteins. Lipids, however, play an important part in signaling processes, membrane stability, and distribution of cell surface receptors in polarized cells. Lipids are also found as anchors of peripheral membrane proteins, many of which are involved in membrane biosynthesis, transport, and signaling (e.g. G-proteins). Lipid disorders are associated to both defective biosynthesis and faulty degradation of specialized lipids. The latter is leading to the accumulation of metabolic intermediates that cause toxic effects, usually in neurons. Tay-Sachs and Gaucher disease are caused by defective degradation of gangliosides. Enzyme replacement therapy became available as the first effective treatment for Gaucher disease. The treatment consists of a modified form of the glucocerebrosidase enzyme given intravenously. Some lipids serve as anchors of membrane proteins. Hemoglobinuria (blood in urine) is related to a defective synthesis of the glycosyl phosphatidylinositol (GPI) anchor of an extra-cellular protein that protects red blood cells from chemical damage. Excessive cell damage leads to high concentration of globin in the urine. A defective synthesis of protective myelin sheets around peripheral neurons have been identified in Charcot-Marie-Tooth (CMT) syndrome. CMT is a multifactorial syndrome caused by different proteins and affecting different peripheral neuronal processes. The underlying mechanism of the disease is a dysfunction of the myelination of axonal extensions of neurons. Lack of myelination causes a slow down of neuronal signaling (action potential speed). Different proteins can be affected including the gene encoding for the peripheral myelin protein-22 (PMP22), the gene encoding myelin protein zero (MPZ, a sensorineural peripheral polyneuropathy), the lamin A/C gene (a nuclear protein; mutations cause a wide spectrum of neuromuscular and cardiac lipodystrophies, with the functional consequence of this mutation seemingly associated with a disorganization of the lamina), the gene encoding ganglioside-induced differentiation-associated protein-1 (GDAP1), the small GTPase late endosomal protein RAB7 (trafficking defects), the gene encoding glycyl tRNA synthetase (GARS), and X-linked Charcot-Marie-Tooth disease (CMTX) with proven connexin 32 (Cx32 or beta1) mutation associated with deafness. Sensorineural deafness observed in this family suggests that Cx32 could play an important role in the auditory pathway. Lipids are also involved in mediating inflammatory responses during infections and the coordination of energy homeostasis in the body. Lipids such as prostaglandins and leukotrienes are now recognized as signaling molecules that function as ligands for membrane bound receptors (GPCRs) and thus their presence or absence are inherently important for a healthy life. They are derived from polyunsaturated fatty acids such as arachidonic acid and eicosapentanoic acid. As signaling molecules, prostaglandin and leukotriene derivatives may be well suited as drugs to treat diseases by activating pathways that can compensate for loss of function. A new glaucoma drug activates an F2 receptor that stimulates extracellular matrix metallo proteases (MMPs) that cause tissue degradation clearing a pathway for fluid drainage and thus reducing built-up intraocular pressure that over long period of times will damage the retinal surface. Other glaucoma drugs are beta-adrenergic antagonists reducing pressure build up. Lipids Online - Educational Resources in Atherosclerosis
Gene therapy and drug delivery As became evident from the previous chapters, cell membranes are veritable barriers to all sorts of molecules but contain specific transport systems that are under cellular and physiological control. Since both viruses and certain toxins use the intracellular machinery for their replication or cytotoxic activity, respectively, they need a cell entry pathway. To get into their host cells, viruses and toxins bind to cell surface receptors inducing membrane fusion or activating the endocytotic machinery, a physiological up-take mechanism of cells for the import of large particles into cells. These patho-physiological processes can be mimicked and exploited for the targeted introduction of therapeutic drugs. The same mechanisms are used for genetherapy applications, where healthy genes are (re)introduced into cells of diseased tissues or organs. Three basic mechanisms are most commonly used to develop systems for the targeted delivery of macromolecules into cells: they include liposome fusion, toxin transport, and viral uptake. The problem with toxins and viruses using receptor mediated endocytosis is that this pathway ends in intracellular organelles instead of in the cytoplasm. The solution to this problem is to use various techniques to develop immunotoxins, fusogenic peptides, or artificial viruses with the ability to cross the membranes of these intracellular organelles. Viral envelopes and bacterial toxins are examples of delivery mechanisms that also provide specificity of interaction via cell surface receptor binding. In addition to gene therapy and the introduction of nucleic acids into cells, protein replacement therapy is of growing interest and depends on the functional reconstitution of membrane proteins. Liposome fusion can also be used to introduce either intracellular proteins or cell surface receptors, where the membrane protein is reconstituted into the liposome (vesicle) membrane prior to fusion. Additional ligands can guide the liposomes to specific target cells. Fusogenic proteins such as haemaglutinine in the liposome membrane will increase the efficiency of liposome fusion with cell membranes. Viral vectors: Viral vectors for genetherapy include Adenovirus, Hepatitis B (HBV), and Herpes Simplex 1 (HSV-1), all of which replicate in the nucleus of human cells and insert their genetic material into the genome of their host cells. Genetically modified viral particles can thus be used to introduce a gene of interest into a particular cell type, tissue, or organ. Toxins: Two widely used toxins as vehicles for drug or DNA transport into cells are diphtheria toxin (DT) and cholera toxin (CT). Both toxins are composed of two subunits, a membrane transport subunit and a cytotoxic subunit. The latter is transported across lysosomal membranes triggered by acidic conditions into the cytoplasm where it inhibits protein biosynthesis. Linking drug or DNA molecules to the cytotoxic subunit may result in the import of chimeric molecules into the cytoplasm of target cells. Fusion process: A variety of self-assembling complexes exhibiting specific binding properties can be used to import large quantities of a drug or DNA of interest into cells via receptor mediated endocytosis. Self-assembly systems include cationic (positively charged) liposomes, lipoproteins, or other synthetic polymers coated with polylysine-conjugates that promote receptor specific attachment to cell surfaces. The presence of multiple positive charged surface groups promotes the binding of negatively charged DNA molecules to these synthetic transport vehicles. In all cases, the particle undergoes a fusion with the targeted cell membrane releasing its contents into the cytoplasm of the cell. Peptides have been shown to be important as small molecules interacting cell membranes. This interaction is mostly used as defense mechanism leading to disruption of the attacked membrane and the subsequent death of the cell or organisms. These peptides are amphipathic forming large and unregulated pores in membranes depleting ion gradients. Many naturally occurring ionophoric peptides have been described. They function often as antibiotics and thus bioengineering strategies are employed to mimic the function of these peptides and find novel antibiotics that are able to overcome the resistance many bacteria are evolving against common antibiotics used in hospitals. Peptide nanotubes are designed based on cyclic characteristics found in the naturally occurring antimicrobial Gramicidin peptides. Because these cyclic peptides are designed to stack on top of each other forming short tubes perforating cell membranes, combinatorial rearrangements of peptide rings with differing amino acid composition can quickly be screened to adapt antibiotics to specific bacterial clones that emerge in hospitals and agricultural settings.
Copyright © 2000-2013 Lukas K. Buehler |
|||||||||||||||||||||||||||